With the Ideal Implant, women will no longer have to choose between peace of mind and a great result when it comes to breast augmentation. Dr. Larry Nichter explains.
Saline and Silicone, Safety and Performance

Women have so far had essentially just two breast implant fill choices: saline or silicone.
Silicone implants have distinct advantages over saline implants in terms of performance: they wrinkle less and conform to a more natural breast shape, and also have a softer feel that is more breast-like. However, silicone implants have gained a reputation—possibly undeserved—for being less safe than saline implants. Despite the fact that there is no known toxicity of silicone gel breast implants, the possibility of a “silent rupture,” undetectable except by MRI, has been enough to make many women opt for saline implants or wait for a better product to come along. The time will be here most likely within a year or so with the advent of the Ideal Implant, the name given to a new design saline hybrid implant. It has the natural feel of silicone and safety of saline.
Saline implants, though providing peace of mind by being perceived as safer than silicone, often do not create a result that seems as natural. Wrinkling, scalloping, a globular shape, and water balloon-like feel, and increased risk of capsular contracture have been the trade-off for peace of mind with breast implants. The Ideal Implant solves many if not all of these concerns.
The Ideal Implant has both
The Ideal Implant is one of the major technological advances to come along in the past few decades in implant manufacture. Using a novel design with internal baffles, the saline implant is manufactured to achieve a similar feel and performance comparable to a silicone implant. Approximately 95% of both patients and their surgeons expressed satisfaction at the current two-year data point by the FDA. The Ideal Breast Implant is soon to be released in the US market, hopefully with in the year.
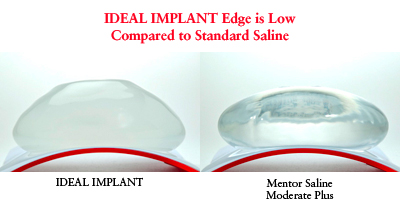
As one of the lead FDA study investigators, I have personally followed my Ideal Implant patients for more than two years. The vast majority of my patients are thrilled with the results. My follow up exams indicate that in most cases they have the feel more like silicone than saline.
When placed on a convex surface similar to the curve of the chest wall, the Ideal Implant conforms to a more natural breast shape; its edge lies lower and its outward surface does not collapse. This is because engineers designed the Ideal Implant with three nested baffle shells, which are unattached and perforated. This makes the saline move slowly between the compartments, giving it gel-like characteristics and a feel more like that of a natural breast.
The Ideal breast implant uses no new materials or manufacturing processes—only tried and tested ones—and is made completely in the United States. The manufacturing process is automated to ensure uniform thickness of the membrane shell, unlike the hand-dipped ones that are used for breast implant manufacturers by other companies. Perhaps most important, the President of the Company, Dr. Robert Hamas, indicated that this implant will only be made available to board certified plastic surgeons by the American Board of Plastic Surgery. To date, the other saline breast implant companies in the US, Allergan and Mentor, sell their product essentially to any MD or DO whether they are a plastic surgeon or not, and even to non-surgeons without board certification. This gives an added advantage to the consumer knowing that when they have an Ideal breast implant placed it will be done by someone well trained and competent—by a board certified plastic surgeon.
The Ideal breast implant is currently undergoing FDA trial studies in 502 women, the second-year results were recently released and are very promising. The capsular contracture rate, for example, at the two-year follow up mark of the current FDA study indicates a dramatic decrease of capsular contracture. To date, the Ideal breast implants show significantly lower rates of capsule contracture and wrinkling, as well as high patient satisfaction. Deflations from early manufacturing defects were identified and addressed, and manufacturing processes have been refined.
Though it has yet to be released to the general public, the Ideal Implant is shaping up to be a contender in the open market with traditional saline and silicone implants.
—Dr. Larry Nichter, MD, FACS
Additional Source: The Ideal Implant website
To receive updates about the ideal implant or to be placed on Dr. Nichter’s waiting list for the procedure, please contact our office using the form below.
[si-contact-form form=’2′]
