 Women wanting breast augmentation have until now faced a dilemma: a choice between safer but less natural-feeling saline implants, and more natural-feeling silicone implants which are perceived to come with health risks.
Women wanting breast augmentation have until now faced a dilemma: a choice between safer but less natural-feeling saline implants, and more natural-feeling silicone implants which are perceived to come with health risks.
The Ideal Implant®, a new breast implant soon to be made available to the public, promises to change all of this. The Ideal Breast Implant is a saline implant with the natural feel of a silicone implant made possible by its innovative double-lumen design. This design consists of a series of nested shells which control the flow of liquid inside the implants, giving them a more realistic feel than standard saline implants.
The Ideal Implant has completed its two-year trials with stunning results. Of the 472 women who came in for two-year follow-up examinations, patient satisfaction for those who received the Ideal Implant as their first implants was 94.3%. Satisfaction among women who received Ideal Implants as replacements for their previous ones was 92.3%. Surgeon satisfaction with the results was also high (96.5% for primary implants and 93.4% for replacements).
Dr. Larry Nichter, one of the surgeons assessing the new implants for the FDA, comments very favorably on the new breast implants in a press release by the American Society for Aesthetic Plastic Surgery:
 “The two-year clinical data from this study show that the Ideal Implant may provide a good alternative to current saline- or even silicone gel-filled implants. One of our most significant and unexpected findings was the low rate of capsular contracture for the investigational, double-lumen implant compared to current single-lumen saline implants.”
“The two-year clinical data from this study show that the Ideal Implant may provide a good alternative to current saline- or even silicone gel-filled implants. One of our most significant and unexpected findings was the low rate of capsular contracture for the investigational, double-lumen implant compared to current single-lumen saline implants.”
The Ideal Implant’s two-year statistics for capsular contracture were better than those for regular saline implants at one year, and none of the small number of deflations were caused by shell fold flaws.
As a further guarantee of quality and safety, the Ideal Implant will only be available through plastic surgeons who are certified by the American Board of Plastic Surgery, which is the only plastic surgery board recognized by the American Board of Medical Specialties. This means that women who choose the Ideal Implant will automatically be choosing from among the best plastic surgeons in the United States.
To receive updates about the ideal implant or to be placed on Dr. Nichter’s waiting list for the procedure, please contact our office using the form below.
[si-contact-form form=’2′]
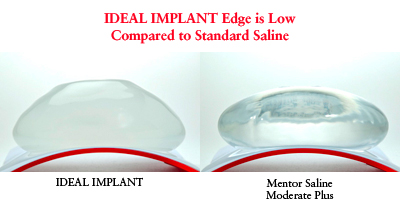 Natural feel is not the only thing women care about. The FDA approved intact silicone gel implants as safe. However, if a gel implant ruptures the implant and any gel should be removed including the surrounding capsule (capsulectomy). IDEAL® IMPLANT offers women natural feel without the risk of silent rupture. Women value the advantages of IDEAL® IMPLANT over silicone gel:
Natural feel is not the only thing women care about. The FDA approved intact silicone gel implants as safe. However, if a gel implant ruptures the implant and any gel should be removed including the surrounding capsule (capsulectomy). IDEAL® IMPLANT offers women natural feel without the risk of silent rupture. Women value the advantages of IDEAL® IMPLANT over silicone gel:

 Breast implant maker Sientra has announced their Capsular Contracture Care Program, the first of its kind in the industry. The C3 Program provdes breast implant patients with a guarantee beyond the standard manufacturer’s warranty. From Sientra:
Breast implant maker Sientra has announced their Capsular Contracture Care Program, the first of its kind in the industry. The C3 Program provdes breast implant patients with a guarantee beyond the standard manufacturer’s warranty. From Sientra: Women wanting breast augmentation have until now faced a dilemma: a choice between safer but less natural-feeling saline implants, and more natural-feeling silicone implants which are perceived to come with health risks.
Women wanting breast augmentation have until now faced a dilemma: a choice between safer but less natural-feeling saline implants, and more natural-feeling silicone implants which are perceived to come with health risks. “The two-year clinical data from this study show that the Ideal Implant may provide a good alternative to current saline- or even silicone gel-filled implants. One of our most significant and unexpected findings was the low rate of capsular contracture for the investigational, double-lumen implant compared to current single-lumen saline implants.”
“The two-year clinical data from this study show that the Ideal Implant may provide a good alternative to current saline- or even silicone gel-filled implants. One of our most significant and unexpected findings was the low rate of capsular contracture for the investigational, double-lumen implant compared to current single-lumen saline implants.”